HAEMOLYTIC
DISEASE OF THE NEW-BORN
BY
####### ######## #######
MLS/11/###
A
SEMINAR WORK PRESENTED IN PARTIAL FULFILLMENT OF THE REQUIREMENT FOR THE AWARD
OF BACHELOR OF
MEDICAL
LABORATORY SCIENCE
(B.MLS)
DEPARTMENT
OF MEDICAL LABORATORY SCIENCE
HAEMATOLOGY
UNIT
FACULTY
OF HEALTH SCIENCE
MADONNA
UNIVERSITY
ELELE
RIVERS STATE
SUPERVISOR:
MR. EZE
CO-ORDINATOR:
DR. ADEGOKE ADEBAYO
MARCH 2016
CERTIFICATION
This is to certify that this seminar
work title “Haemolytic disease of the new-born” was done by Udoutun Rosemary
Patrick (MLS/11/140) met the regulation governing the award of Medical
Laboratory Science, Madonna University, Nigeria and is hereby approved for its
contribution to knowledge.
MR. EZE
…………………………
………………………..
Supervisor
Signature Date
DR.
ADEGOKE ADEBAYO
………………………....
………………………..
Co-ordinator
Signature Date
ASSOC.
PROF. NNATUANYA .I.
…………………………..
………………………..
H.O.D
Signature Date
DEDICATION
This work is dedicated to God Almighty and
Blessed Virgin Mary
ACKNOWLEGEMENT
My
special thanks goes to God Almighty, Jesus Christ the son and Blessed Virgin
Mary for the graces bestowed upon me. My acknowledgement goes to my supervisor
Mr. Eze in a very special way for his wonderful advice and knowledge he
impacted in me to make sure that am successful in studies and also for his
supervision. My heartfelt gratitude goes to my parents, Mr/Mrs Patrick .M.
Udoutun and also my siblings who were always there to support me morally,
spiritually, financially and otherwise. I also want to acknowledge my HOD.
ASSOC. PROF. Isaac Nnatuanya, my lecturers and friends for their moral and
academic support.
SUMMARY
Haemolytic
Disease of the New-born (HDN) also known as Erythroblastosis fetalis is an
immune haemolytic anaemia that is characterized by the presence of
immunoglobulin G (IgG) antibodies in the maternal circulation, directed against
paternally derived antigen present in the foetus causing destruction of baby's
red blood cells. Symptoms such as; Oedema, new-born jaundice, enlarged liver
and spleen, anaemia, still birth may occur in severe cases. The vast majority
of HDN is caused by Rhesus “D” antigen; it may be caused by ABO
incompatibility, and also due to other blood group incompatibility. IgG class
antibody is mostly implicated in HDN because of its ability to cross the
placenta. The mother usually develops antibodies following an early stimulus
before affecting the foetus; this exposure can be during first pregnancy.
During antenatal visit, screening tests are done to predict pregnancies at risk
of HDN. The administration of RhIg (Rhogam) a human anti-D globulin to all
unsensitised Rh-negative mothers after abortion, miscarriage and deliver of an
Rh-Positive infant prevents sensitization to Rhesus factor. Treatment can be
with intrauterine transfusion, induction of early
pregnancy, phototherapy,
exchange transfusion, and depending on
the form of HDN.
CHAPTER ONE
1.0
INTRODUCTION
Haemolytic Disease of the New-born(HDN)
also known as Erythroblastosis fetalis or haemolytic disease of the foetus is
an immune haemolytic anaemia that is characterized by the presence of
immunoglobulin G (IgG) antibodies in the maternal circulation, directed against
a paternally derived antigen present in the foetal/neonatal red cells that
cause haemolysis in the fetus by crossing the placenta and sensitizing red
cells for destruction by the macrophages in the fetal spleen thereby destroying
the new-born baby's blood cells very quickly and causing symptoms such as;
Oedema(swelling under the surface of the skin),New-born jaundice, enlarged liver and spleen, anaemia,
still birth may occur in severe case(Hadley AG and Vox Sang,1998).
The vast majority of HDN is caused by Rhesus
“D” antigen. Several foetal rhesus antigen may cause alloimmunization (c, C, D,
e, E) and this occurs with the Kell, Duffy, ABO, MNS and other blood group systems.
Pregnancies at risk of HND are those in which an Rh D-negative mother becomes
pregnant with an Rh D-positive child (the child having inherited the D antigen
from the father). The mother's immune response to the fetal D antigen is to
form antibodies against it (anti-D). These antibodies are usually of the IgG type,
the type that can cross the placenta into the foetal circulation. HDN can also
be caused by an incompatibility of the ABO blood group. It arises when a mother
with blood type O becomes pregnant with a fetus with a different blood type
(type A, B, or AB). The mother's serum contains naturally occurring anti-A and
anti-B, which tend to be of the IgG class and can therefore cross the placenta and
haemolyses fetal RBCs. HDN due to ABO incompatibility is usually less severe
than Rh incompatibility. (Urbaniak, et al.,
2000, Garratty, et
al., 2004).
Antenatally,
the presence of anti-D antibodies in the mother is detected by indirect coomb’s
test which is usually carried out on all Rhesus negative mothers during their subsequent
antenatal visit. Once the presence of maternal anti-D has been confirmed, the
next step is to determine whether the fetal RBCs are a target, i.e., confirm
the Rh status of the fetus. If the father is homozygous for the D allele (D/D),
the fetus will be D positive. If however the father is heterozygous (D/d),
there is a 50:50 chance that the foetus maybe D positive or negative, and the only
way to know the blood type for sure is to test a sample of fetal cells taken
from the amniotic fluid or umbilical cord. If the fetus is Rh D-positive, the
pregnancy is carefully monitored for signs of HDN. Monitoring includes regular
ultrasound scans of the fetus and monitoring of the amount of anti-D in the
mother's serum (Urbaniak, et al.,
2000, Garratty, et al., 2004). If
anti-D is not found in the mother's serum, it is likely that she has not been
sensitized to the Rh D antigen. The risk of future sensitization can be greatly
reduced by prophylactic administration of anti-D Ig (RhoGAM) at 28weeks and
again at 32-34weeks of pregnancy and if needed within 72hours of delivery to
"mops up" any fetal RBCs that may have leaked into the maternal
circulation.
Incidence
of HDN is dependent on the population who are Rh-D negative. In most tropical countries
like Africa and Asia, HDN is likely to be caused by ABO incompatibility than Rhesus
incompatibility due to low number of Rhesus negative women (Garratty et al.,
2004).
.1
HISTORY
Haemolytic
disease of the new-born used to be a major cause of fetal loss and death among new-born
babies. The first description of HDN is thought to be in 1609 by a French midwife;
Louise Bourgeois (Bowman JM, 1998). In 1609, Bourgeois assisted at a twin
birth: the first twin was markedly hydropic and practically dead at delivery,
whereas the second developed rapidly worsening jaundice within a few hours and
died three days after birth. For the next 300 years, many similar cases were
described in which new-borns failed to survive (Gruslin, et al., 2011, Cohen and Waltham, 2009).
In
1932 the three most characteristic clinical signs of Rh HDN, that is, hydrops fetalis,
severe neonatal jaundice and delayed anaemia of the new-born, were recognised
as expressions (with different incidences and severities) of a single
pathological process, confirming the hypothesis proposed by Diamond and his colleagues,
they believed that the pathogenesis of the disease involved a defect of the
erythron (Bowman, 1999). It was not until the 1950s that the underlying cause
of HDN was clarified that the new-born’s red blood cells (RBCs) are being
attacked by antibodies from the mother. The attack begins while the baby is
still in the womb and is caused by an incompatibility between the mother's and
baby's blood (Gruslin, et
al., 2011, Cohen and Waltham, 2009)
In
the 1960s, trials in the United States and the United Kingdom tested the use of
therapeutic antibodies that could remove the antibodies that cause HDN from the
mother's circulation. The trials showed that giving therapeutic antibodies to
women during their pregnancy largely prevented HDN from developing (Urbaniak, et al., 2000). In the 1970s, routine
antenatal care included screening of all expectant mothers to find those whose
pregnancy may be at risk of HDN, and giving preventative treatment accordingly.
This has led to a dramatic decrease in the incidence of HDN, particularly
severe cases that were responsible for stillbirth and neonatal death. (Garratty,
et al., 2004).
1.2
GENETICS
Although
the Rh antibody is the most common cause of severe haemolytic disease of the new-born,
other alloimmune antibodies belonging to Kell (K and k), Duffy (Fya), Kidd (Jka
and Jkb), and MNSs (M, N, S, and s) systems do cause severe haemolytic disease
of the new-born(Van der Schoot CE, et al.,2003).
The Rh blood group system uses Fisher-Race nomenclature, and the Rh gene
complex consists of 3 genetic loci each with 2 major alleles. They code for 5
major antigens denoted by letters, C, c, E, e, and D. Rh blood group antigens
are inherited as determined by at least 2 homologous but distinct
membrane-associated proteins. Two separate genes: RhCE and RhD, located on the
short arm of chromosome 1, encode Rh proteins. Production of 2 distinct
proteins from the RHCE gene is due to alternative splicing of messenger RNA. Rh
gene complex is described by 3 loci, and, therefore, 8 gene complexes are
possible.
These
complexes are as follows; CDe, cde, CDE, cDe, Cde, cdE, cDE, and CdE.
Expression is limited to RBCs, with an increasing density during their
maturation, unlike the ABH system, which exists in a wide variety of tissues.
Rh antigen is not expressed on RBC progenitors. Of individuals who are Rh
positive, 45% are homozygous (CDe/CDe), and 55% are heterozygous (CDe/cde) for
the RhD gene (Singleton BK, et al.,
2000). The Rh-negative phenotype represents absence of D protein on RBCs and
most commonly results from deletion of the RHD gene on both chromosomes.
However, the RHD gene has significant heterogeneity, and several inherited
mutations and rearrangements in its structure can result in a lack of
expressions of the RhD phenotype as well. Important examples of such mutations
include the RHD pseudogene and RHD-CE-D hybrid gene (Flegel WA, 2011)
Beyond
the 5 major antigens, more than 100 antigenic variants of Rh group system have been
identified. Individuals with these weak-D phenotypes comprise of 2 populations:
first group (90%) that expresses normal but reduced quantities of D antigen on
the RBC surface and most cannot be sensitized to produce anti-D. However, the
second group (remaining 10%) known as partial-D (e.g., Cw, Du) that express
partial D epitopes on RBC surface and can make anti-D and rarely experience foetal
haemolytic disease of the new-born. The partial D phenotype results from amino
acid substitution in the active RhD epitope (Moise KJ, 2008). Most women with
partial-D phenotype are classified as Rh negative on routine testing and are
candidates for Rhesus immune globulin (RhIG). However, Rh-negative infants born
to Rh-negative women should undergo testing to detect the partial-D phenotype
so that RhIG can be administered in the event of weak expression.
1.3 PATHOPHYSIOLOGY
Occurrence
of HDN as a result of red blood cell alloimmunization secondary to pregnancy
involves three key stages. First, a paternally derived red blood cell antigen
foreign to the mother must be inherited by the foetus. Second, the volume of
foetal red cells that gain access to the maternal circulation must be
sufficient to stimulate an immune response in the particular individual.
Finally, maternal antibodies to foetal red cells must gain transplacental
access and cause immune destruction of sensitized red cells by Fc
receptor-bearing effector cells in the foetus and neonate(Bidyut, et al .,2010).
 |
| Diagram showing the pathophysiology of HDN |
Figure 1: Diagram showing the
pathophysiology of HDN. Source:McGraw-Hill Companies, Inc.
Rhesus
alloimmunisation begins with red blood cells from a rhesus positive foetus
crossing the placental barrier during pregnancy and delivery, and entering the
maternal blood circulation. A rhesus positive father and a rhesus negative
mother are required for this situation to develop. The incompatible antigens
introduced result in a primary immune response and stimulate the production of
maternal antibodies (Kumar and Regan; 2005).
In
a first pregnancy, Rh sensitization is not likely to cause problems; it becomes
a problem in a future pregnancy with another Rh positive baby. When the next
pregnancy occur, the mother’s antibodies cross the placenta then reacts with
the corresponding antigen to fight the Rh positive cells in the baby’s body
that the baby has inherited from the father which is foreign to the mother. Hence,
antigen antibody interaction occurs. Sensitization of baby’s red blood cell
(RBC) by mother’s IgG antibody causes the baby’s RBC to be destroyed, thereby
causing haemolysis. These antibody-coated RBCs are then removed from fetal
circulation by the macrophages of the spleen and liver. This haemolysis results
in anaemia, hyperbilirubinaemia and the production of excessive erythroid
tissue in the liver, spleen, bone marrow, skin and placenta. In severe cases, multi-organ
dysfunction and hypoproteinaemia can develop. The severity of anaemia depends on
the amount of mother’s antibody, its specificity, its avidity, and other characteristics.
Anaemia will stimulate bone marrow to produce more RBC including immature RBC,
which is then released to fetus circulation. This is also known as
Erythroblastosis fetalis.
HDN
is often classified into three categories, on the basis of the specificity of
the causative IgG antibody: D haemolytic disease caused by anti-D alone or,
less often, in combination with anti-C or anti-E, other haemolytic disease
caused by antibodies against other antigens in the Rhesus system or against
antigens in other systems; anti-c and anti-K are most often implicated, and ABO
HDN caused by anti-A or anti-B (Brecher M.E. 2005).
CHAPTER TWO
LITERATURE REVIEW
2.1
EPIDEMIOLOGY
2.1.1
FREQUENCY
The
incidence of haemolytic disease of the new-born depends on the proportion of
the population who are RhD negative. This varies within ethnic minorities but,
in the UK, it is highest in the Caucasian population (approximately 16%).
Before immunoprophylaxis was available, HDN affected 1% of all new-borns and
was responsible for the death of one baby in every 2,200 births but incidence
of Rh sensitization has declined from 45 cases per 10,000 births to 10.2 cases
per 10,000 total births, with less than 10% requiring intrauterine transfusion
(Chavez et al., 1991). Currently,
anti-D is still one of the most common antibodies found in pregnant women,
followed by anti-K, anti-c, and anti-E. Of those foetus that require
intrauterine transfusions, 85%, 10%, and 3.5% were due to anti-D, anti-K, and
anti-c, respectively (Lindenburg et al., 2012). ABO
incompatibility frequently occurs during the first pregnancy and is present in
approximately 12% of pregnancies, with evidence of foetal sensitization in 3%
of live births. Less than 1% of births are associated with significant haemolysis.
2.1.2
MORTALITY AND MORBIDITY
Only
3 antibodies are associated with severe foetal disease: anti-Rh-D, anti-Rh-c,
and anti-Kell (K1). Nearly 50% of the affected new-borns do not require
treatment, have mild anaemia and hyperbilirubinemia at birth, and survive and
develop normally. Approximately 25% are born near term but become extremely
jaundiced without treatment and either die or become severely affected by
kernicterus. The remaining 25% of affected new-borns are severely affected in
utero and become hydropic (Bowman JM, Creasy RK et al., 1999).
2.1.3 RACE
Incompatibility
involving Rh antigens (anti-D or anti-c) occurs in about 10% of all pregnancies
among whites and blacks; in contrast, it is very rare in Asian and African
women because of the low number of Rh negative women.
2.1.4
SEX
Fetal
sex plays a significant role in the degree of response to maternal antibodies.
An apparent 13-fold increase is observed in fetal hydrops in RhD-positive male
foetus compared with female foetus in similarly sensitized pregnancies (Kaplan
M, Na'amad M, et al., 2009)
2.2
PROGNOSIS
The
severity of this condition can vary. Some babies have no symptoms. In other
cases, problems such as hydrops can cause the baby to die before, or shortly
after birth. Severe HDN may be treated before birth by intrauterine blood
transfusion (Gruslin AM et al., 2011;
Cohen and Waltham; 2009).
2.3
SIGNS AND SYMPTOMS
HDN
can destroy the new-born baby's blood cells very quickly, which can cause
symptoms and each infant may experience symptoms differently. Complications can
range from mild to severe.
Before birth:
·
With amniocentesis (process of
withdrawal of amniotic fluid with a needle for the purpose of analysis) the
amniotic fluid may have a yellow colouring and contain bilirubin.
·
Hydrops Fetalis: This will occur as the
baby's organs are unable to handle the anaemia. The heart begins to fail and
large amounts of fluid build-up in the baby's tissues and organs. In severe
forms this can include petechiae and purpura. The infant may be stillborn or
die shortly after birth.
·
Ultrasound of the foetus shows enlarged
liver, spleen, or heart and fluid build-up in the foetus' abdomen.
After
birth:
·
Jaundice: Due to the destruction of red
blood cells, there’s a usually elevated bilirubin level. After delivery
bilirubin is no longer cleared (via the placenta) from the neonate's blood,
Infants unable to get rid of the bilirubin so bilirubin builds up in the
blood(hyperbilirubinemia) and other tissues and fluids of the infant's body
resulting in JAUNDICE. Symptoms of jaundice (yellowish skin and yellow
discoloration of the whites of the eyes) increase within 24hours after birth;
there is the possibility of acute or chronic kernicterus (damage to Brain
centres).
·
Anaemia: Anaemia limits the ability of the
blood to carry oxygen to the infant's organs and tissues and lead to breathing difficulties
to the infant. Profound anaemia can cause high-output heart failure, with pallor,
and respiratory distress.
·
Babies with hydrops fetalis have severe
oedema of the entire body and are extremely pale. They often have breathing
problems.
2.4
CAUSES
HDN
may develop when a mother and her unborn baby have different blood types called
"incompatibility"(Gruslin, et al., 2011; Cohen and Waltham,2009). The
mother produces substances called antibodies that attack the developing baby's
red blood cells. Antibodies produced by the mother potentially causes HDN and is
antibody of IgG class, this antibody is known to cause various degree of
complications to the foetus if the foetus has RBCs containing the corresponding
antigen. IgG class antibody is important in HDN because their ability to cross placenta.
The 2 main common cause of HDN are:
Ø Rhesus
incompatibility
Ø ABO
incompatibility
Ø HDN
can also be caused by other alloantibodies and this is uncommon. Most
frequently involved antibodies of Kell, Duffy, Kidd and MNS blood group system.
2.5: HAEMOLYTIC DISEASE OF THE NEWBORN
CAUSED BY RHESUS INCOMPATIBILITY (RHESUS ISSOIMUNIZATION)
In 1941, Levine and co-workers
first described the involvement of the Rhesus factor in erythroblastosis
fetalis (Levine et al 1941). Rhesus incompatibility is the
most severe form of HDN and sometimes fatal. This form of HDN happens when
mother who is rh-D negative is bearing an Rh-D positive foetus. D positivity of
the foetus is due to genetically inheritance of D from father. Anti-D is the
commonest antibody in Rh system that causes HDN although other Rh antigens such
as c, C, E, and e, can also cause problems. If the father is heterozygous for
the Rh D deletion, there is a 50% chance of the foetus being D-negative. If the
father is homozygous for the Rh D gene, the foetus will definitely inherit the
D antigen. Rh D negative mother lacks a functional Rh D gene and so does not
produce the D antigen, and may be immunized by D-positive foetal blood.
2.5.1:
PATHOGENESIS
Isoimmune
haemolytic disease from D antigen is approximately three times more frequent
among white persons than among black persons. When small quantities of Rh-D foetal
blood containing D antigen inherited from an Rh-positive father enter the maternal
circulation during pregnancy, with spontaneous or induced abortion, or at
delivery, antibody formation against D antigen may be induced in the unsensitised
Rh-negative recipient mother. Once sensitization has taken place, considerably smaller
dose of antigen can stimulate an increase in antibody titre.
Haemolytic
disease rarely occurs during a first pregnancy because transfusion of
Rh-Positive foetal blood into the Rh-Negative mother occurs near the time of delivery,
too late for the mother to become sensitized and transmit antibody to her baby
before delivery. The fact that 55% of Rh-Positive fathers are heterozygous (D/d)
and may have Rh-negative offspring and that foetal-to-maternal transfusion
occurs in only 50% of pregnancies and has reduced the chance of sensitization.
But once a mother has been sensitized, in subsequent pregnancies with
Rh-positive baby, the baby is likely to have haemolytic disease; the severity
of Rh illness worsens with successive pregnancies. The injection of anti-D
gamma globulin (RhoGAM) into the mother immediately after the delivery of each
Rh-Positive infant has been a successful strategy to reduce Rh haemolytic
disease.
2.5.2:
CLINICAL MANIFESTATIONS
A
wide spectrum of haemolytic disease occurs in affected infants born to
sensitized mothers, depending on the nature of the individual's immune response.
The severity of the disease may range from only laboratory evidence of mild
haemolysis (15% of cases) to severe anaemia with compensatory hyperplasia of erythropoietic
tissue leading to massive enlargement of the liver and spleen. When the compensatory
capacity of the haematopoietic system is exceeded, profound anaemia occurs and
results in pallor, signs of cardiac decompensation, massive anasarca, and
circulatory collapse.
Ø HYDROPS FETALIS: This
clinical picture of excessive abnormal fluid in two or more foetal compartment (skin,
pleura, pericardium, placenta, peritoneum, amniotic fluid) termed hydrops
fetalis which frequently results in death in utero or shortly after birth due
to profound anaemia and circulatory failure.
 |
| Image of a neonate suffering from hydrops fetalis. |
Figure
2: Image of a neonate suffering from hydrops fetalis. Source:
Adamimages.com
The severity of hydrops fetalis is
related to the level of anaemia and degree of reduction in serum albumin which
is due in part to hepatic dysfunction. Alternatively heart failure may increase
right heart pressure, with subsequent development of oedema and ascites,
failure to initiate spontaneous effective ventilation becomes pulmonary oedema,
after a successful resuscitation, severe respiratory distress may develop.
Petechiae, purpura and thrombocytopenia may also be present in severe cases as
a result of decreased platelet production.
Ø JAUNDICE:
Jaundice may be absent at birth because of placental clearance of lipid soluble
conjugated bilirubin, but in severe cases, bilirubin pigments can stain the amniotic
fluid, cord, and Vernix caseosa yellow. Jaundice is generally evident on the
first day of life because the infant's bilirubin conjugating and excretory
systems are unable to cope with the load resulting from massive haemolysis.
Indirectly reacting bilirubin accumulates postnatal and may reach extremely
high levels and present a significant risk of bilirubin encephalopathy and the
risk of development of kernicterus is very high.
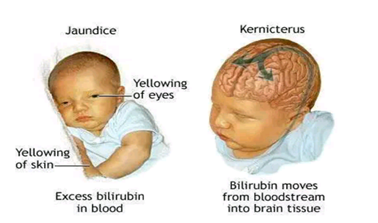 |
| Diagram of a neonate suffering from Jaundice and Kernicterus.
Figure 3: A diagram of a neonate suffering from Jaundice and
Kernicterus.source:Adamimages.com
|
Infants
born after intrauterine transfusion for prenatally diagnosed Erythroblastosis
may be severely affected because the indications for transfusion are evidence
for already severe diseases utero (hydrops, foetal anaemia).such infants
usually have very high cord level of bilirubin reflecting severity of
haemolysis. Infants treated with intraumbilical vein transfusion in utero also
have benign. Anaemia from continuing haemolysis may be masked by the previous intrauterine
transfusion and the clinical manifestations of erythroblastosis may be superimposed
on various degrees of immaturity resulting from induced premature delivery.
2.5.3:
LABORATORY TEST
Maternal blood
·
ABO and Rh grouping: ABO and Rh grouping are ordered the first time
a pregnant woman sees a physician. The father may also be tested at this time.
If the woman is Rh negative and the father is heterozygous for the D antigen,
the possibility of the infant being D positive is 50%.
·
Antibody screen and identification: Antibody screen is
done to detect whether sensitization to D antigen has developed, if the initial
antibody screen is positive, the antibody is identified and a titre is
determined. Antibody titration is done to help the obstetrician determine the
severity of HDN and the need for foetal monitoring (ultrasound, amniocentesis, and
cordocentesis).
·
The
Kleihauer–Betke test or flow cytometry on a postnatal maternal blood sample can
confirm that fetal blood has passed into the maternal circulation and can also
be used to estimate the amount of foetal blood that has passed into the
maternal circulation.
·
The indirect Coombs test is used to
screen blood from antenatal women for IgG antibodies that may pass through the
placenta and cause haemolytic disease of the new-born.
Foetal blood (or umbilical cord
blood)
·
Cord blood: Perform ABO and Rh grouping.
·
If HDN is suspected, do direct antiglobulin (DAT)
test. If the infant’s cells are heavily coated with maternal antibody, there
may be a reaction in the Rh control as well as the Rh test. It may not be
possible to get a proper Rh type or even ABO without first performing heat
elution of the antibody.
·
If the DAT is positive, or if the DAT is negative
but the infant shows symptoms, elute the antibody from the cord blood cells and
run a panel to determine the identity of the antibody. Compare to the mother’s
antibody.
·
If the mother has anti-A, anti-B or anti-AB, and
the infant is Group A or B, test the elute against screening cells, A1 and B
cells. In ABO-HDN the cord cells may be DAT negative but the cord serum may
have anti-A, anti-B or anti-A,B. Test the serum against A1, B, and O red cells
by indirect antiglobulin method.
·
Full blood count.
·
Bilirubin (total and indirect).
2.5.4:
LABORATORY FINDINGS
·
Before treatment, direct coombs test
result is usually positive and anaemia is usually present.
·
The cord blood haemoglobin content
varies and is usually proportional to the severity of the disease; with hydrops
fetalis it may be as low as 3-4g/dL. Alternatively, despite haemolysis, it may
be within the normal range because compensatory bone marrow and extra medullary
haematopoiesis.
·
Blood smear typically shows polychromsia
and a marked increase in nucleated red blood cells.
·
The reticulocyte count is increased.
·
The white blood cell count is usually
normal but may be elevated in some cases; thrombocytopenia may develop in
severe cases.
·
Biochemical test: Cord bilirubin is
generally between 3-5 mg/dL, the direct reacting bilirubin content is usually elevated,
especially if there was an intrauterine transfusion. Indirect reacting bilirubin
content rises rapidly to high levels in the first 6hrs of life.
·
After intrauterine transfusions, cord
blood may show a normal haemoglobin concentration, negative direct coombs test result,
predominantly type O Rh-negative adult red blood cells and relatively normal
smear findings.
2.5.5:
DIAGNOSIS
Definitive
diagnosis of Erythroblastosis fetalis requires demonstration of blood group
incompatibility and corresponding antibody bound to the infant's red blood
cells and can be done through prenatal and postnatal test.
Antenatal
diagnosis
In
Rh-negative women with history of previous transfusions, abortions,
miscarriages and pregnancy should suggest the possibility of sensitization.
Expectant parent's blood types should be tested for potential incompatibility
and the maternal titre of IgG antibodies to D antigen should be assayed at 12-16,
28-32 and 36weeks of gestation. The presence of elevated antibody titres at the
beginning of pregnancy, a rapid rise in titre suggests significant haemolytic disease,
although the exact titre correlates poorly with the severity of the disease. If
the mother is found to have antibody against D antigen at a titre of 1:16 or
greater at any time during subsequent pregnancy, the severity of foetal disease
is monitored by Doppler ultrasonography of the middle cerebral artery and also
by percutaneous umbilical blood sampling (PUBS) if indicated.
Information
obtained from ultrasonography and PUBS is required for the assessment of the foetus.
Ultrasonographic signs of hydrops include organomegaly (liver, spleen, and
heart), double bowel wall sign and placenta thickening. Real time
ultrasonography predicts fetal well-being by means of the biophysical profile
which includes; fetal breathing movement, gross body movement, fetal tone,
reactive fetal heart rate, qualitative amniotic fluid volume, whereas Doppler
ultrasonography assesses fetal distress by demonstrating increased vascular resistance
in fetal arteries (middle cerebral). PUBS is performed to determine the fetal
haemoglobin levels and to transfuse packed red blood cells in those with anaemia
(Hct 25-30%).
Amniocentesis
is used for detection of bilirubin level, fetal lung maturity profile,
biophysical profiles and middle cerebral artery peak systolic velocity. It
allows spectrophotometric measurement of deviation in optical density (OD) at
450nm due to bilirubin level in amniotic fluid, which reflects fetal RBC haemolysis.
Ultrasonographically guided, transabdominal aspiration of amniotic fluid may be
performed at 18-20 weeks of gestation. Amniocentesis procedure should be
carried out only after careful ultrasound placental localization. If it must be
carried out without the use of ultrasound, a suprapubic approach may be less
likely to encounter the placenta. Amniocentesis is an invasive procedure with
risk to both the foetus and mother, including fetal death, bleeding, bradycardia,
worsening of alloimmunization, premature rupture of membranes, preterm Labour and
chorioamnionitis.
Postnatal
diagnosis
Immediately
after the birth of an infant to an Rh-negative mother, blood from the umbilical
cord or from the infant should be examined for ABO blood group, Rh type, Hct
and haemoglobin levels, and reaction to the direct coombs test. If the direct coombs
test result is positive, a baseline serum bilirubin level should be examined and
a commercially available RBC panel should be used to identify antibodies present
in the mother's serum, both tests being performed not only to establish the diagnosis
but also to ensure selection of the most compatible blood for exchange transfusion,
if necessary. The direct coombs test result is usually positive in clinically
affected infants and may remain so for few days up to several months.
2.5.6:
TREATMENT
Before delivery of an unborn infant: If HDN is detected
during pregnancy (before delivery) that is Rh HDN cases, it will be treated using;
·
Intrauterine transfusion (IUT),
·
Induction of early delivery if the
foetus develops complication.
Intrauterine Transfusion: Intrauterine
transfusion is done if the foetus if affected severely, transfusions will be
done every 1 to 4 weeks until the foetus is mature enough to be delivered
safely. Amniocentesis may be done to determine the maturity of the foetus’s
lungs before delivery is scheduled. It is given to the fetus to prevent hydrops
fetalis and fetal death. This method can be done as early as 17 weeks, although
preferable to wait until 20 weeks. After multiple IUTs, most of the baby’s
blood will be D negative donor blood, therefore, the direct antiglobulin test
will be negative, but the indirect antiglobulin test will be positive.
 |
| Image of a neonate receiving Intrauterine Transfusion. |
Figure
4: Image of a neonate receiving Intrauterine Transfusion. Source:
Adamimages.com
After
IUTs, the cord bilirubin is not an accurate indicator of rate of haemolysis or
of the likelihood of the need for post-natal exchange transfusion. Intrauterine
transfusion procedure is done firstly by maternal and fetal sedation with
diazepam and by fetal paralysis with pancuronium.
After
the mother's abdomen is cleaned with an antiseptic solution, she is given a
local anaesthetics injection to numb the abdominal area where the transfusion
needle will be inserted. Medication is also given to the foetus to temporarily
stop foetal movement. An ultrasound image is obtained to determine the position
of the fetus and placenta. Ultrasound is used to guide the needle through the
mother's abdomen into the foetus’s abdomen or an umbilical cord vein. A compatible
blood type (usually type O, Rh-negative) is delivered into the foetus’s
abdominal cavity or into an umbilical cord blood vessel. The mother is usually
given antibiotics to prevent infection. She may also be given oncolytic
medication to prevent labour from beginning, though this is unusual. The risk
of these procedures is now largely dependent on the prior condition of the
fetus and the gestational age at which transfusion is commenced.
Transfusion
should achieve a post transfusion Hct of 45-55% and can be repeated every
3-5weeks. Indications for delivery include: pulmonary maturity, fetal distress,
complications of PUBS. The survival rate for intrauterine transfusion is 89%. Complications
include rupture of the membranes, preterm delivery, infection, fetal distress requiring
emergency caesarean section and perinatal death.
Induction
of early pregnancy: Early
delivery is recommended if the foetus develops complications. If the fetus has
mature lungs, labour and delivery may be induced to prevent worsening of HDN.
AFTER DELIVERY OF A LIVE-BORN INFANT: The
birth should be attended to by a physician skilled in neonatal resuscitation. Fresh,
low titre, group O, leuko-reduced and irradiated Rh-negative blood cross
matched with mother’s serum should be immediately available. If clinical signs
of severe haemolytic anaemia are seen at birth, immediate resuscitation and supportive
therapy, temperature stabilization, and monitoring before proceeding with exchange
transfusion may save some severely affected infants. Supportive therapies
like;
·
Correction of acidosis with 1-2 mEq/kg
of sodium bicarbonate
·
A small transfusion of compatible packed
red blood cells to correct anaemia
·
Volume expansion for hypotension,
especially in those with hydrops
·
Provision of assisted ventilation for
respiratory failure.
EXCHANGE TRANSFUSION
Exchange
transfusion in which the infant’s blood is removed in small amounts usually 5
to 10 ml at a time and is replaced with a compatible blood (Rh-negative blood),
is a standard mode of therapy for treatment of severe hyperbilrubinemia that is
unresponsive to phototherapy, and it is the treatment of choice for severe hyperbilirubinemia
and hydrops fetalis caused by Rh incompatibility. Exchange transfusion removes
the sensitized erythrocytes, lowers the serum bilirubin levels to prevent
bilirubin encephalopathy, corrects the anaemia, prevent cardiac failure.
Indications for exchange transfusion include rapidly increasing serum bilirubin
levels and haemolysis despite aggressive phototherapy. The criteria for exchange
transfusion in preterm infants vary according to associated illness factor.
For
exchange transfusion, fresh whole blood is typed and cross matched to the mother's
serum. Heparin or citrate-phosphate-dextrose-adenine solution may be used as an
anticoagulant. If the blood obtained before delivery, it should be taken from a
type O, Rh-negative donor with a low titre of anti-A and anti-B antibodies and
it should be compatible with the mother's serum by the indirect coombs test.
After delivery, blood should be obtained from an Rh-Negative donor whose cells
are compatible with the infant's and the mother's sera; when possible, type O donor
cells are generally used. The amount of donors blood used is usually double the
infant’s blood volume, which is approximately 85 ml/kg body weight. The double-volume
exchange transfusion replaces approximately 85% of the neonate’s blood.
An exchange transfusion is a sterile surgical procedure,
with aseptic techniques a polyvinyl catheter is inserted into the umbilical
vein and threaded into the inferior vena cava. Depending on the infant's
weight, 5 to 10 ml of blood is withdrawn within 15 to 20 minutes and the same
volume of donor's blood is infused until the targeted volume (double the estimated
blood volume) is reached. If he donor's blood has been citrate, calcium
gluconate may be given after the infusion of each 100ml of donor's blood to
prevent hypocalcaemia. During exchange transfusion, blood is gradually warmed
and maintained at a temperature between 35 and 37°c.i It is kept well mixed by
gentle squeezing or agitation of the bag to avoid sedimentation; otherwise the
use of supernatant serum with low Rbc count at the end of the infusion will
leave the infant anaemia. The infant's stomach should be emptied before
transfusion to prevent aspiration, and body temperature should be maintained
and vital signs monitored during the process. Acute complications noted in 5-10%
of infants include; transient vasospasm, thrombosis, apnea with bradycardia
requiring resuscitation and death. Infection risks include CMV, HIV, and
hepatitis.
After
exchange transfusion, the bilirubin level must be determined at frequent
intervals (4-8hours) because bilirubin may rebound 40-50% within hours.
Repeated exchange transfusion should be carried out to keep the indirect
fraction from exceeding the levels.
Benefits of exchange transfusion
·
Removal of bilirubin
·
Removal of sensitized RBCs
·
Removal of incompatible antibody
·
Replacement with compatible RBCs
·
Suppression of erythropoiesis: reduced production
of incompatible RBCs
2.5.7:
LATE COMPLICATIONS
Infants
who have haemolytic disease or who have had an exchange or an intrauterine
transfusion must be observed carefully for the development of anaemia and
cholestasis. Late anaemia may be haemolytic or hypo regenerative.
Treatment with supplemental iron, blood transfusion or erythropoietin may be
indicated. A mild GVH reaction may manifest as diarrhoea, rash, hepatitis or eosinophilia.
Insipissated
bile syndrome refers to the rare occurrence of persistence citrus in
association with significant elevations in direct and indirect bilirubin levels
in infants with haemolytic disease. The cause is still unclear but the jaundice
clears spontaneously within a few weeks or months.
Portal
vein thrombosis and portal hypotension may occur in children who have been
subjected to exchange transfusion as new-born infants. It is probably
associated with prolonged, traumatic, or septic umbilical vein catheterization.
2.5.8: PREVENTION OF RHESUS ISSOIMMUNIZATION
Immunization of the D antigen can be prevented by the
administration of Rh immunoglobulin either before or shortly after exposure to
Rh-positive cells. This dose of immunoglobulin has three mechanisms of action:
antigen blocking (i.e. competitive inhibition) by attaching to or covering
antigenic sites on the Rh-positive red cells; clearance and antigen deviation;
central inhibition by the generation of antigen-specific suppressor cells
(Schott J.R, et al 1999). Despite
this; some investigators believed that the precise mechanism is still unclear
(Koelewijn JM, et al 2009). The
percentage of anti-D immunization decreased to 0.7-2.5% in the various
countries after the introduction of anti-D immunoprophylaxis (Engelfriet CP et al 2003). Initial studies proved that
the postpartum administration of a single dose of anti-D immune globulin to
susceptible RhD-negative women within 72 h of delivery reduced the alloimmunization
rate by 90% (Bidyut K, et al 2010).
Rh immunoglobulin (RhIg) is a concentrate of
predominantly IgG anti-D derived from pools of human plasma. A full dose of
anti-D (300-µg-1500 IU) is sufficient to counteract the immunizing effects of
15 ml of D-positive red cells; this corresponds to approximately 30 ml of foetal
whole blood (Brecher M.E. 2005). RhIg prophylaxis is administrated to
unimmunized Rh-negative women following events that might allow foetal red
cells to enter the maternal circulations, i.e. delivery, spontaneous or
therapeutic abortion, ectopic pregnancy, amniocentesis, chorionic villus
sampling, cordocentesis, antepartum haemorrhage, blunt abdominal trauma, and
foetal death (Engelfriet CP, et al
2003). Antepartum RhIg at or after 28 weeks is also recommended (Brecher M.E,
2005; Bidyut K, et al 2010;
Engelfriet CP, et al 2003). Massive
FMH can lead to immunization as the standard dose of RhIg fail to cover this
excess of amount. A screening test such as the rosette technique should be
used, and, if positive, quantification of the haemorrhage must be done by
Kleihauer-Betke test or by flow cytometry (Brecher, 2005; Engelfriet, et al 2003; Harmening, 2005).
The failure of anti-D Ig prophylaxis related to
increased FMH and /or insufficient anti-D Ig levels (Koelewijn, et al 2009)
2.6 HAEMOLYTIC DISEASE OF THE NEWBORN
CAUSED BY ABO INCOMPATIBILITY
Haemolytic
disease can also occur when the major blood groups antigen of the foetus are
different from those of the mother. The major blood groups are A, B, AB, and O.
It is the most common cause of HDN with most cases being mild. Significant
problems with ABO incompatibility occur mostly with babies whose mothers have O
blood type and where the baby is either A or B blood type. The mother’s history
of prior transfusions or pregnancies seems unrelated to the occurrence and
severity of the disease, thus ABO HDN may occur in the first pregnancy and in
any subsequent pregnancies (Harmening D.M; 2005).
Antibodies
in the plasma of one blood group (except the AB group, which contains no
antibodies) will produce agglutination when mixed with antigens of a different blood
group. The agglutinated donor cells become trapped in peripheral blood vessels,
where they haemolyse, releasing large amount of bilirubin into the circulation.
The most common blood group incompatibility in the neonate is between a mother
with O blood group and an infant with A and B blood group as seen below;
MARTENAL BLOOD GROUP
|
INCOMPATIBLE FETAL BLOOD
GROUP
|
O
|
A or B
|
B
|
A or AB
|
A
|
B or AB
|
Figure
5: Maternal-foetal ABO incompatibilities. Source: expertconsult.com
Naturally
occurring anti-A or anti-B antibodies already present in the maternal
circulation cross the placenta and attach to the foetal red blood cells causing
haemolysis. Usually the haemolytic reaction is less severe than in Rh
incompatibility. Unlike Rh reaction, ABO incompatibility may occur in the first
pregnancy. The risk significant haemolysis in subsequent pregnancies is thought
to be unchanged from the first (Luchtman-Jones, et al 1997).
There
seem to be two main reasons for low incidence and severity of ABO HDN despite
considerable foetal-maternal ABO incompatibility; first, the A and B antigens
are not fully developed at birth, and second, A and B substances are not
confined to the red cells so that only a small fraction of IgG anti-A and
anti-B which cross the placenta combines with the infants red cells (Anstee, 2005).
Prenatal screening for maternal ABO antibodies can demonstrate the presence of
IgG antibody but do not correlate well with the extent of foetal RBCS
destruction. Therefore, detection of ABO HDN is best done after birth (Duguid,
1997; Harmening, 2005).
2.6.1:
CLINICAL MANIFESTATIONS
Most
cases are mild, with jaundice being the only clinical manifestation. The infant
is not generally affected at birth; pallor is not present, and hydrops fetalis
is extremely rare. The liver and the spleen are not generally enlarged.
Jaundice usually appeared during the first 24 hrs. Rarely, it may become severe
and symptoms and signs of kernicterus develop rapidly.
2.6.2:
DIAGNOSIS
A
presumptive diagnosis is based on the presence of ABO incompatibility, a weakly
to moderately positive direct coombs test result, and spherocytes in the blood
smear, which may at times suggest the presence of hereditary spherocytosis.
Hyperbilirubinemia is often the only other laboratory abnormality. The haemoglobin
level is usually normal but may be as low as 10-22 g/dL. Reticulocytes may be
increased to 10-15%, with extensive polychromasia and increased numbers of
nucleated Rbcs. In 10-20% of affected infants, the unconjugated serum bilirubin
level may reach 20 mg/dL or motor unless phototherapy is administered.
2.6.3:
SEROLOGICAL DIAGNOSIS
·
Direct coomb test on cord blood is usually positive in the
first 24hours of life
·
Direct on blood drawn after the first
24hours of life is usually negative
·
Elution: the presence of ABO antibody in
elute from the baby’s blood usually suggest ABO HDN
·
Haemoglobin level is usually normal or
near normal.
2.6.4:
TREATMENT
Phototherapy
may be effective in lowering serum bilirubin levels. In severe cases, IVIG
administration can reduce the rate of haemolysis and the need for exchange
transfusion. Exchange transfusion with type O blood of the same Rh type as the
infant may be needed in some cases to correct dangerous degrees of anaemia or hyperbilirubinemia.
Indications for this procedure are similar to those previously described for
haemolytic disease may require transfusion of packed Rbcs at several weeks of
age because of slowly progressive anaemia. Post discharge monitoring of
haemoglobin is essential in new-born with ABO haemolytic disease.
For
phototherapy at the hospital, a light box and/or fiber-optic blanket directs
fluorescent light onto the jaundiced baby. The baby lies in a bassinet or an
enclosed crib (incubator) while light is absorbed into the skin.
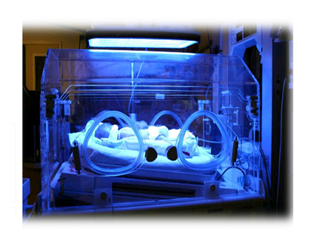 |
| phototherapy in an incubator. |
Figure
6: phototherapy in an incubator.
The
blue wave length of natural light is absorbed by the bilirubin chemical in his
skin when it is exposed to the light. The light wave changes the shape of the
bilirubin molecules (the process is called photo-isomerization). This
different– shaped bilirubin molecule is more easily taken out of the baby's
body by his kidneys. The baby’s urine will turn a dark, dusty colour during
phototherapy. The light treatment is safe and effective. The baby's eyes are
usually covered for comfort because if he stares at that bright blue light long
enough he get headache. The light is not dangerous to his eyes, or ours. Also,
babies under phototherapy treatment need to sleep, and they seem to sleep
better if their eyes are covered. The more a baby's skin is exposed to the blue
light, the better it works. The light treatment does not work through clothes
or diapers. After a few hours of light exposure, you can tell that the yellow
is disappearing from his skin, but is still there under the diaper and the
eye-shades.
Feeding
your baby is an essential part of treating yellow jaundice. Milk going through
your baby's digestive system is the signal to his liver that tells it to turn
on. So, your baby must be taken out from under the phototherapy lights to get a
good feeding at least every three hours. If your milk hasn't come in yet, and
the baby needs phototherapy, you may be asked to supplement your baby's
feedings with your pumped milk or formula until the baby is nursing well at the
breast. If the baby isn't under the lights, the bilirubin keeps going up. If
the baby doesn't get fed, the liver isn't turning on. So there must be a
balance between the two important parts of treatment, phototherapy and
feedings.
Natural
sunlight contains all the wavelengths of light, so it has plenty of the blue wavelengths
that convert the bilirubin molecule into the easier-to-pee- out form. But it
must be light through a window, not outside. The window glass filters out the
ultraviolet light that causes sunburn, so that the baby will not have sunburn. Two
or three times a day, fifteen to twenty minutes at a time of bright sunlight
shining on your baby's bare-naked skin really will help keep yellow jaundice
down. It sort of depends on what kind of windows people have at your home.
2.7:
OTHER FORMS OF HAEMOLYTIC DISEASE
Blood
group incompatibilities other than Rh or ABO account for <5% of haemolytic
disease of the new-born.
·
Haemolytic disease of the new-born due
to anti-Kell alloimmunization
·
Other blood group antibodies (Kidd,
Lewis, Duffy, MN, P and others) but the occurrence is very rare.
2.9:
SENSITIZATION DURING PREGNANCY
Sensitization
to an antigen occurs when the immune system encounters an antigen for the first
time and mounts an immune response. The three most common models in which a woman
becomes sensitized toward (i.e., produces IgG antibodies against) a particular
antigen are; Fetal-maternal haemorrhage, Blood transfusion, Immune response to
antigen A and B.
2.9.1: Foetal-maternal haemorrhage
Pregnancy presents special immunohematology problems
for the transfusion service. The mother may exhibit alloimmunization to
antigens on foetal cells, and the foetus may be affected by maternal antibodies
provoked by transplacental passage, are produced. Most women who become alloimmunized
do so as a result of FMH of less than 0.1 ml (Scott J.R. et al 1999, Bidyut K. 2010). Several studies suggested that
abnormalities of pregnancy, methods of delivery, and therapeutic procedures are
possible causes of FMH. The American College of Obstetrics and Gynaecology
(ACOG) has defined pregnancies with one of the following circumstances as being
at high risk for FMH of 30 ml or more: antepartum foetal death, antepartum
bleeding, intrauterine manipulation, placenta prevail, abruption placenta, and
caesarean section. Manual removal of the placenta (Sebring E.S and Polesky H.F
1990), amniocentesis, threatened abortion, abdominal trauma, anaemic infant
(Schott J.R. etal 1999), therapeutic
and spontaneous abortion, ectopic pregnancy (Bidyut K. etal 2010) represent additional causes of FMH.
The
risk of sensitization to the Rh D antigen is decreased if the foetus is ABO
incompatible. This is because any foetal cells that leak into the maternal
circulation are rapidly destroyed by potent maternal anti-A and/or anti-B,
reducing the likelihood of maternal exposure to the D antigen.
2.9.2:
Blood Transfusion
ABO
blood group system and the D antigen of the Rhesus (Rh) blood group system
typing are routine prior to transfusion. Suggestions have been made that women
of child bearing age or young girls should not be given a transfusion with
Rhc-positive blood or Kell1- positive blood to avoid possible
sensitization, but this would strain the resources of blood transfusion
services, and it is currently considered uneconomical to screen for these blood
groups. HDN can also be caused by antibodies to a variety of other blood group
system antigens, but Kell and Rh are the most frequently encountered.
2.9.3:
Immune response to antigen A and B
Another
sensitization model can occur in women of blood type O. The immune response to
A and B antigens, that are widespread in the environment, usually leads to the production
of IgM or IgG anti-A and anti-B antibodies early in life. Women of blood type O
are more prone than women of types A and B to making IgG anti-A and anti-B
antibodies, and these IgG antibodies are able to cross the placenta. For
unknown reasons, the incidence of maternal antibodies against type A and B
antigens of the IgG type that could potentially cause haemolytic disease of the
new-born is greater than the observed incidence of "ABO disease."
About 15% of pregnancies involve a type O mother and a type A, B, or AB child;
only 3% of these pregnancies result in haemolytic disease due to A/B/O
incompatibility. In contrast to antibodies to A and B antigens, Rhesus
antibodies are generally not produced from exposure to environmental antigens.
3.1:
HEAMOLYTIC DISEASES IN SUBSEQUENT PREGNANCIES
Initially
if a woman has been sensitized in the past, it is very important to be closely
monitored by an experienced Obstetrics/Gynaecologist during any future
pregnancy with an Rh-positive partner. The maternal anti-D that is formed at the
time of sensitization is of the IgM type, which cannot cross the placenta. In
subsequent pregnancies, a repeat encounter with the Rh D antigen stimulates the
rapid production of type IgG anti-D, which can be transported across the
placenta and enter the fetal circulation. Once in the fetal circulation, anti-D
attaches to the Rh D antigens found on the fetal RBCs, marking them to be
destroyed. The rate of haemolysis determines whether the nature of HDN is mild,
moderate, or severe.
·
In Severe cases: Fetal hydrops refers to
the widespread and serious destruction fetal red blood cells. This condition
leads to the fetus developing severe anaemia which can lead to heart failure,
total body swelling, respiratory distress, liver and/or spleen enlargement,
jaundice and highly elevated bilirubin levels. One or more fetal blood
transfusions may be needed before birth. In very severe cases of Rh disease the
fetus may need a blood exchange, which replaces the majority of the new-born’s
blood with donor blood, usually type O-Negative. Other treatment procedures
that may be implemented include use of phenobarbital before delivery and
infusions of albumin after delivery to help reduce bilirubin levels, as well as
phototherapy treatments.
·
In Moderate cases: there is destruction of
a larger numbers of fetal red blood cells, moderate anaemia, possible
development of an enlarged liver and jaundice is looked for closely. Preterm
delivery may be necessary to remove the fetus form the hostile environment and
they may require a blood transfusion before and/or after birth.
·
In Mild cases: it may involve limited
destruction of the fetal red blood cells and could result in mild fetal anaemia.
Usually the fetus can be carried to term and requires little or no special
treatment. However, some jaundice may occur after delivery. This lighter level
of Rh-Disease is usually seen in a first pregnancy after sensitization has
occurred.
·
After delivery: infants who have
suffered a severe Rh incompatibility and are experiencing extreme cases of
jaundice are at the greatest risk for developing Kernicterus; which is a
neurological syndrome caused by deposits of bilirubin in the tissue of the
brain. This syndrome is characterized by symptoms which may include the loss of
the new-born’s startle reflex, a decreased amount of activity and poor feeding.
Additional more serious symptoms include a shrill sounding cry, a bulging
fontanel and possibly seizures. If the infant survives Kernicterus, they may
later experience poor muscle tone, seizures, tone specific hearing loss and sometimes
decreased mental ability. Kernicterus is a very serious condition that demands
a physician or specialist who is experienced in Rh incompatible pregnancies and
their possible complications.
4.1:
PREDICTION OF HAEMOLYIC DISEASE
Prediction
of haemolytic is a process of forecasting the possibility of haemolytic disease
occurring under specific conditions.
·
During the first antenatal visit, all
pregnant women should have samples collected, ideally at 10-16 weeks gestation
for ABO and Rhesus typing and for the presence of red cell alloantibodies.
Whether the woman is rhesus positive or negative, further blood samples should
be taken at 28weeks for rechecking the ABO and Rhesus group and further red
cell alloantibodies (Thompson et al, 2003).
·
Rhesus negative mothers with antibodies
are screened for rise in antibody titre at intervals of 20, 28, 36, and 38
weeks of gestation.
·
When red cell antibodies are detected in
Rhesus negative mothers, further testing of maternal blood should be undertaken
to determine the specificity, concentration, origin and level of antibody or
antibodies, and the likelihood of HDN. Anti-D, anti-c and anti-K are the
antibodies most often implicated in causing haemolytic disease severe enough to
warrant antenatal intervention. The antibody titre is checked at 2weeks
interval, if constant it is disregarded but if it keeps rising to a critical
point, induction maybe recommended at 38weeks, if the titre is indicative or if
the amniotic fluid bilirubin is high.
·
For those in first pregnancy who are
Rhesus negative, where a clinically significant antibody capable of causing
HDN, particularly anti-D, anti-c or anti-K, is present in a maternal sample,
determining the father’s phenotype provides useful information to predict the
likelihood of a fetus carrying the relevant red cell antigen. The complexities
of paternal testing and the potential for misidentification of the father need
to be acknowledge [National Collaborating Centre for Women’s and Children’s
Health, 2003] the husbands are screened for ABO and Rhesus group, if the
husband is negative, then the pregnancy is not at risk but if the husband is
Rhesus positive, the pregnancy might be at risk and there might be problems in
subsequent pregnancies and may qualify her for RhoGAM injection.
5.1: CAUSATIVE
ANTIBODIES
Ø ANTI D: Despite the use of Rh
immunoglobulin, anti-D is still a common antibody identified in alloimmunized
women (O.Geifman-Holtzman et al 1995,
Bidyut K. etal 2010, Howard H etal 1998).
Anti-D shows a wide spectrum of severity. Not all D positive infants born
to mothers with anti-D in their serum are affected by HDN. About 50% of Rh
positive new-born infants with Rh (D) HDN are so mildly affected that they
require no treatment. Anti-D is the most important after anti-A and B. It is
mostly IgG with IgG1 and IgG3 as predominant subclasses. Naturally occurring IgG
anti-D that were only detectable in an autoanalyser, were reported to be
relatively common. Anti-C, c, E, e shared many of the characteristics of
anti-D. Anti-c is clinically the most important Rh antibody after anti-D and
may cause severe HDN. (Daniel G 2002).
A study by Tovey LA (1980) concluded that the
number of pregnant women with antibodies other than anti-D exceeded those with
anti-D (Tovey LA 1986). Severe HDN caused by antibodies other than anti-D is
associated with anti-K, and anti-c (Koelewijn JM etal 2008, Brecher M.E. 2005,
Bidyut K. etal 2010). Significant rhesus antibodies that can cause HDN include:
Anti-c, Anti-C, Anti-CW, Anti-E, Anti-e, Anti-Rh29,
and Anti-Rh17.
Ø Kell antibodies:
Kell alloimmunization is the second major cause of foetal anaemia, with a
reported and still increasing incidence. In the foetus, Kell antibodies cause
suppression of Kell-positive erythroid precursor cells, a mechanism different
from D haemolytic disease where the A antibodies cause destruction of RBCS
(Marije M.K etal 2008). Another feature of anti-K HDN is a poor correlation
between the severity of the disease and the titre of antibody in the mother
serum (Klein .H.J. & Anstee D.J 2005).Significant kell antibodies include:
Anti-K, Anti-k, Kpb, and Anti-Jsb.
Ø Antibodies
of the ABO system: Anti-A and anti-B.
Ø Antibodies
belong to the other blood group systems: Anti-Fya, Anti-M, Anti-S, Anti-U,
Anti-PP1PK, Anti-JKa, anti-ELO, anti-MAM.
CHAPTER THREE
CONCLUSION
IgG class of antibody is
the most implicated antibody in haemolytic disease of the new-born which is usually possessed by the mother,
this antibody has the ability to cross the placenta and cause various degree of
complications to the foetus if the foetus has corresponding antigens. Maternal
sensitization occurs very late in rhesus negative pregnancy, hence the first
rhesus positive child is usually not affected but subsequent pregnancies with rhesus
positive child will be severely affected. The life span of the infant's red
cells is shortened by destruction through the actions of maternal antibodies
and this usually leads to anaemia, neonatal jaundice, hydrops fetalis and still
birth may occur in severe cases. HDN caused by ABO incompatibility is usually
mild compared to that of Rhesus incompatibility. Laboratory testing plays a
crucial role in the identification of at-risk pregnancies, the diagnosis of the
disease, and the identification of the antibody causing the problem, so that
the proper treatment can be followed. Prophylactically administration of Rhogam
to rhesus negative mothers has decreased the rate of anti-D immunization.
REFERENCES
Anstee,
D. J. (1990). Blood group-active surface molecules of the human red blood cell.
Journal of haemolysis; 58:1-20.
Akhil,
M. and Waldemar A. C. (2011). Haemolytic disease of the new-born. In: Robert K,
Waldo E, editors. Nelson textbook of Paediatrics. 19th edition Philadelphia, Pa: Elsevier Saunders; 97:615-619.
Bidyut,
K., velathupillia, R. and Zarco, A. (2010). Red cell alloimmunization. Journal of Obstetrics and Gynaecology; 20(2):47-56.
Bowman,
J. M. (1998). Rh-D haemolytic disease of the new-born. New England Journal of Medicine;
339:1775-1777.
Bowman,
J. M. (1999). Hemolytic disease (erythroblastosis fetalis). Creasy RK, Resnik
R. Maternal-foetal medicine. 4th edition. Philadelphia:
WB Saunders; 23:736-767.
Brecher,
M. E. (2005). Technical Manual. Journal
of American Association of Blood Banks; 45:271-293.
Chavez,
G. F., Mulinare, J. and Edmonds, L. D. (1991). Epidemiology of Rh haemolytic
disease of the new-born in the United States. Journal of American Medical Association; 265(24):3270-3274.
Cohen,
D. W. (2009). Haemolytic disease of the new-born: RBC alloantibodies in
pregnancy and associated serologic issues. Journal
of Britain medical association; 23:67-81.
Daniels,
G. (2002). The clinical significance of blood group antibodies. American
Journal Transfusion Medicine; 12:287-295.
Duguid,
J. K. (1997). Antenatal serological testing and prevention of haemolytic
disease of the new-born. Journal of
Clinical Pathology; 50:193-196.
Ebix
A.D.A.M Images. (n.d.). Retrieved Jan 10, 2016, from Ebix: www.adamimages.com/kernicturus-illustration/P127536/F4.
Ebix
A.D.A.M Images. (n.d.). Retrieved Jan 10, 2016, from Ebix: www.adamimages.com/Illustration/searchResult/1/hydrops%20fetalis.
Engelfriet,
C. P., Reesink, H. W., Judd, W. J., Ulander, V. M., Kuosmanen, M. and Koskinen,
S. (2003). Current status of immunoprophylaxis with anti-D immunoglobulin. Vox Sang; 85:328-37.
Flegel,
W. A. (2011). Molecular genetics and clinical applications for Rhesus factor. Journal
of Transfusion and Apherology Science; 44(1):81-91.
Garratty,
G., Glynn, S. A. and McEntire, R. (2004). ABO and Rh (D) phenotype frequencies
of different racial/ethnic groups in the United States. International journal of Transfusion; 44:703–706.
Geifman-Holtzman,
M., Wojtowycz, K. and Kosmas, R. (1997). Female alloimmunization with
antibodies known to cause haemolytic disease. American journal of Obstetrics Gynaecology; 89: 272-275.
Gruslin,
A. M. and Moore, T. R. (2011). Erythroblastosis fetalis. In: Martin R, Fanaroff
A, and Walsh M, eds. Neonatal-Perinatal Medicine. 9th ed. Philadelphia, Pa: Mosby Elsevier; 234: 456-458.
Hadley,
A. G. (1998). A comparison of in vitro tests for predicting the severity of
haemolytic disease of the foetus and new-born. Journal of blood transfusion science; 74(2):375-383.
Harmening,
D. M. (2005). Modern blood banking and transfusion practices. Journal of haematology; 60:177-181.
Kaplan,
M., Na'amad, M. and Kenan, A. (2009). Failure to predict haemolysis and hyperbilirubinaemia
by IgG subclass in blood group A or B infants born to group O mothers. Journal of Paediatrics; 123(1):132-137.
Koelewijn,
J. M., deHaas, M., Vrijkotte, T. G., vanderSchoot, C. and Bonsel, G. (2009).
Risk factors for RhD immunization despite antenatal and postnatal anti-D
prophylaxis. Britain Journal of Obstetrics
and Gynaecology; 116:1307-1314.
Kumar,
S. and Regan, F. (2005). Management of pregnancies with RhD alloimmunisation. Britain Medical Journal; 330(7502):1255-1258.
Levine,
P., Burnham, M. and Katzin, E. M. (1941). Pathogenesis of erythroblastosis
fetalis: statistical evidence. International
of Science; 94(2442)371-372.
Luchtman-Jones,
L., Schwartz, A. L. and Wilson, D. B. (2006). The blood and hematopoietic
system. In Fanaroff AA, Martin RJ, editors: Neonatal-Perinatal Medicine;
diseases of the Foetus and Infant. 8th
ed. St. Louis, Mo: Mosby; 2:1287-1356.
Marije,
M., Kamphuis, H., Irene, L., Inge, L., van, K., Robertjan, H., Meerman, H.,
Kanhai, Z. and Dick, O. (2008). Implementation of routine screening for Kell
antibodies: does it improve perinatal survival? French journal of Transfusion; 48:953-957.
Moise,
K. J. (2008). Haemolytic disease of the foetus and new-born. Creasy RK, Resnik
R. Maternal-foetal Medicine: Principles and Practice. 6th edition. Philadelphia: WB Saunders; 25:477-503.
National
Collaborating Centre for Women’s and Children’s Health / RCOG Antenatal care.
(2003). Routine care of the healthy pregnant woman. www.rcog.org.uk/resources/Public/pdf/AntenatalCare.
Scott,
J. R., Di-Saia, P. J., Hammond, C. B., Spellacy, W. N. (1999). Danforth’s
Obstetrics and Gynaecology. 8th ed.
Lippincott Williams and Wilkins; 143:144-367.
Sebring,
E. S. and Polesky, H. F. (1990). Fetomaternal haemorrhage incidence, risk
factors, time of occurrence and clinical effects. Journal of Transfusion; 30:344-357.
Singleton,
B. K., Green, C. A. and Avent, N. D. (2000). The presence of an RHD pseudogene
containing 37 base pair duplication and a nonsense mutation in Africans with
the Rh-D negative blood group phenotype. American
journal of Blood. 95(1):12-8.
Thompson,
S., Eggington, J., Dodd, A., Qureshi, R., Turner, E. (2003). Late developing
red cell antibodies in pregnancy. International
journal of Transfusion Medicine; 13:
8-9.
Urbaniak,
S. J. and Greiss, M. A. (2000). Rh-D haemolytic disease of the foetus and the
new-born. American journal of Blood science; 14:44–61.







0 Comments